Clinical Trials
Clinical trials are conducted to collect adequate and well-controlled scientific data regarding the safety and efficacy of new drugs and devices under development. There is a well-defined system in place for bringing new drug or device from lab shelf to pharmacy shelf and this process consist of several steps and stages of approval before a drug or device can be sold in the consumer market, if ever. Clinical trials help to produce high-quality data for healthcare decision making.
Drug and device testing begin with extensive laboratory research which can involve years of experiments in animals and human cells. If the initial laboratory research is successful, researchers submit the data to the respective country regulatory authorities (as IND application) for approval to continue research and testing in humans.
Phase I
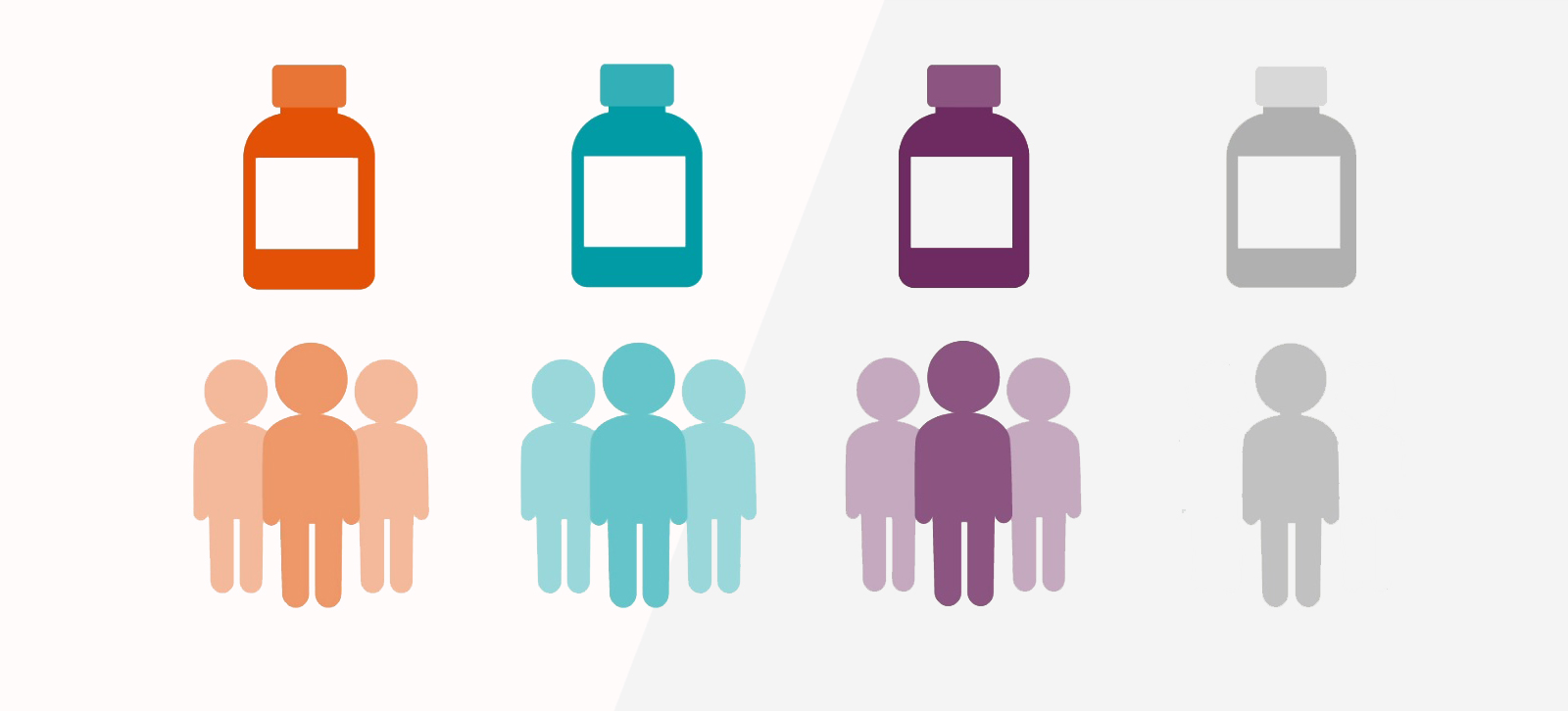
This is the first step wherein a new drug or device is used in humans. These studies primarily assess the safety of a drug or device and additionally pharmacokinetic profile and dose escalations. This initial phase of testing can take several months to complete, usually includes a small number of healthy volunteers (20 to 80). The study is designed to determine the effects of the drug or device on humans including how it is absorbed, metabolized, and excreted. This phase also investigates the side effects that occur as dosage levels are increased. About 70% of experimental drugs pass this phase of testing.
Studies conducted in Phase I typically involve:
- Estimation of initial safety and tolerability
- Pharmacokinetics
- Assessment of pharmacodynamics or early measurement of drug activity.
Phase II
These studies primarily test the efficacy of a drug or device in addition to safety and take several months to two years and involve up to several hundred patients with target indication.
Phase II starts with the initiation of studies in which the primary aim is to explore therapeutic efficacy in patients/participants. Phase II studies are conducted on a group of patients or participants who are selected according to relatively narrow criteria and are closely monitored. Early studies in Phase II are designed to estimate the dose response. Later studies are planned to confirm the dose response. About one-third (33%) of experimental drugs successfully complete both Phases I and Phase II studies.
Phase III
These studies involve randomized and blind testing in several hundred to several thousand patients. Most phase II studies are randomized trials where one group of patients receives the experimental drug, while a second "control" group receives a standard treatment or placebo. Often these studies are "blinded" which means that neither the patients nor the researchers know who has received the experimental drug. This large-scale testing, which can last several years, provides the pharmaceutical company and the regulatory authority with a more thorough understanding of the effectiveness of the drug or device, the benefits and the range of possible adverse reactions. 25% to 30% of drugs that enter Phase III studies successfully complete this phase of testing.
Phase IV
These studies, often called Post Marketing Surveillance Trials, are conducted after a drug or device has been approved for consumer sale. Pharmaceutical companies have several objectives at this stage: (1) to compare a drug with other drugs already in the market; (2) to monitor a drug's long-term effectiveness and impact on a patient's quality of life; and (3) to determine the cost-effectiveness of a drug therapy relative to other traditional and new therapies. Phase IV studies can result in a drug or device being taken off the market or restrictions of use could be placed on the product depending on the findings in the study.

Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants.
Once approved, human testing of experimental drugs and devices can begin and is typically conducted in four phases. Each phase is considered a separate trial and, after completion of a phase, investigators are required to submit their data for approval from the respective country regulatory authorities before continuing to the next phase.